An Exploration of CRISPR in Sickle Cell Disease
Treatment
Spring 2024
 Introduction
Introduction
Sickle cell disease is a group of inherited blood
disorders affecting approximately 100,000 people in the
U.S. It is most common in African Americans, but also
disproportionately affects Hispanic Americans. The
primary problem in sickle cell disease is a mutation in
hemoglobin, a protein in red blood cells that transports
oxygen to the body’s tissues. This mutation causes red
blood cells to appear in a “sickle” shape. These mutated
red blood cells restrict blood flow in vessels and limit
oxygen delivery to the body’s tissues, leading to severe
pain and organ damage called vaso-occlusive events
(VOEs) or vaso-occlusive crises (VOCs). The recurrence
of these crises can lead to life-threatening
disabilities and/or early death.
A relatively new technology that research scientists use to selectively modify the DNA of living organisms, CRISPR, has just been implemented into sickle cell treatment. CRISPR was adapted for use in the laboratory from naturally occurring genome editing systems found in bacteria. On December 8, 2023, the first ever CRISPR gene therapy treatments were approved by the FDA for use in patients over the age of 12. There are so many potential benefits such as reducing the need for frequent and time consuming hospital visits, reducing pain, and improving overall quality of life. Nicole Verdun, M.D., director of the Office of Therapeutic Products within the FDA’s Center for Biologics Evaluation and Research writes, “Gene therapy holds the promise of delivering more targeted and effective treatments, especially for individuals with rare diseases where the current treatment options are limited”. With that being said, as the use of Cas9 in treatment is so new, there is so much we do not know about it. It is extremely important to take the many potential risks into account. Accordingly, this controversial topic leads to the question, is it ethical to use CRISPR as a treatment for sickle cell disease, and what exactly is at stake?
A relatively new technology that research scientists use to selectively modify the DNA of living organisms, CRISPR, has just been implemented into sickle cell treatment. CRISPR was adapted for use in the laboratory from naturally occurring genome editing systems found in bacteria. On December 8, 2023, the first ever CRISPR gene therapy treatments were approved by the FDA for use in patients over the age of 12. There are so many potential benefits such as reducing the need for frequent and time consuming hospital visits, reducing pain, and improving overall quality of life. Nicole Verdun, M.D., director of the Office of Therapeutic Products within the FDA’s Center for Biologics Evaluation and Research writes, “Gene therapy holds the promise of delivering more targeted and effective treatments, especially for individuals with rare diseases where the current treatment options are limited”. With that being said, as the use of Cas9 in treatment is so new, there is so much we do not know about it. It is extremely important to take the many potential risks into account. Accordingly, this controversial topic leads to the question, is it ethical to use CRISPR as a treatment for sickle cell disease, and what exactly is at stake?
 Sickle Cell Treatment Today
Sickle Cell Treatment Today
The most common treatment of sickle cell today is blood transfusions. When anemia, a low blood cell count, is severe, conditions including splenic sequestration, acute chest syndrome, and aplastic crisis are life threatening if not treated with blood transfusions. These transfusions increase the number of normal red blood cells in the body, increasing the supply of oxygen to the body.
An argument for CRISPR in sickle cell disease treatment are the benefits of eliminating downsides of today’s most common treatments, such as blood transfusions. While blood transfusions can help reduce the frequency and severity of sickle cell-related complications, they do not completely eliminate the symptoms. Alloimmunization, the development of antibodies against transfused blood, transmission of infectious diseases such as hepatitis or HIV, and allergic reactions can occur as a result of repeated transfusions. Also, finding compatible blood for transfusions can be challenging and may require extensive screening and matching procedures.
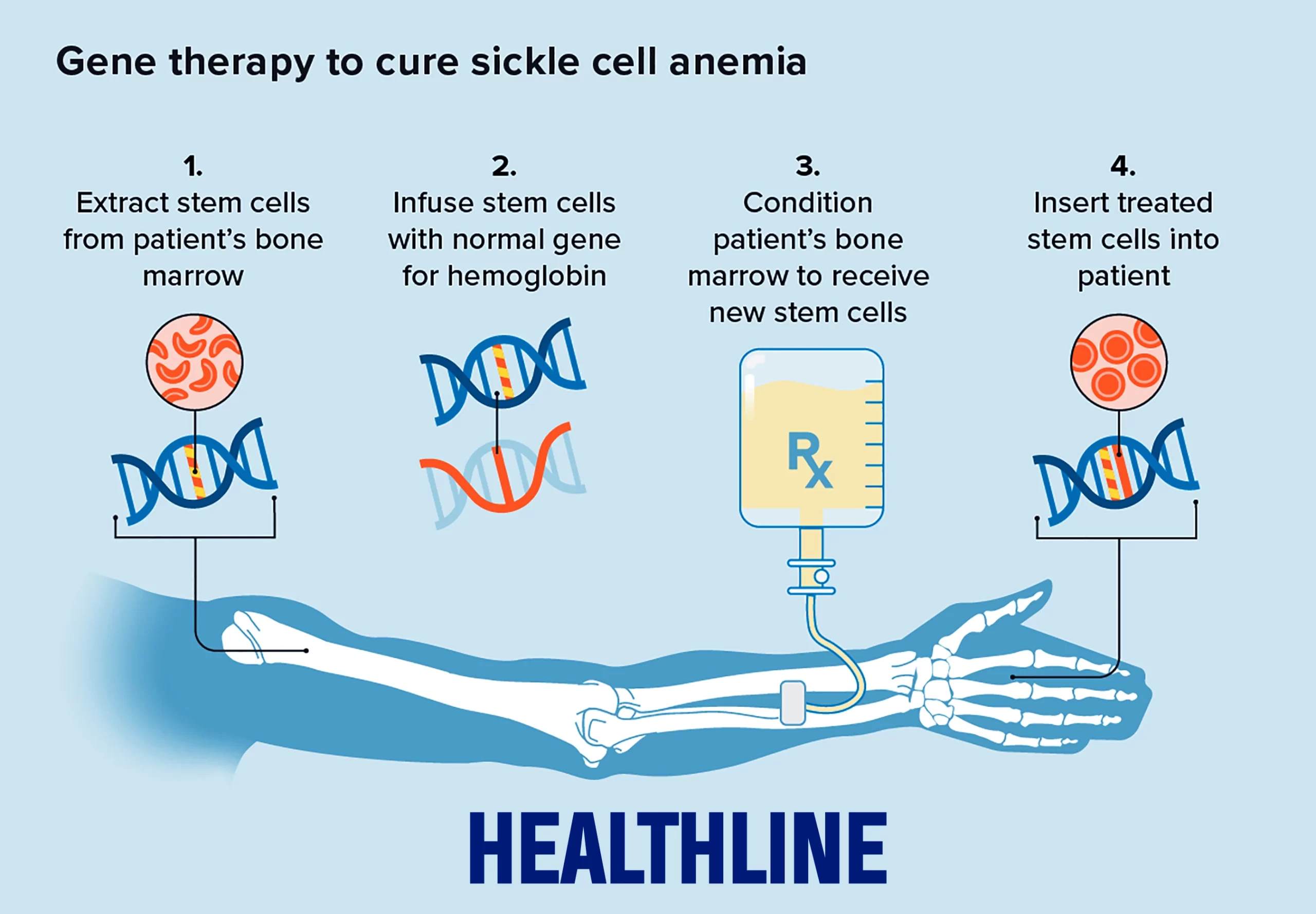
Current CRISPR Therapies At this point, two gene therapies involving the use of Cas9 for treating sickle cell disease have been approved by the FDA for patients 12 years of age and older with recurring vaso-occlusive crises, Casgevy (also called exa-cel) and Lyfgenia. Casgevy is a cell-based gene therapy created by the companies Vertex and CRISPR Therapeutics. The treatment involves mobilizing a patient's own bone marrow stem cells from the blood. The stem cells are edited at a specific region of the BCL11A gene which prevents the production of fetal hemoglobin (HbF), a type of hemoglobin that facilitates oxygen delivery. In patients with SCD, increased levels of HbF prevent the sickling of red blood cells. The reduction of BCL11A gene transcription in the patient’s RNA leads to an increase of HbF production, thus providing functioning hemoglobin. The modified blood stem cells are transplanted back into the patient where they attach and multiply within the bone marrow. The second approved treatment, Lyfgenia, is similar to Casgevy, but is not as common. The patient’s blood stem cells are genetically altered to produce HbAT87Q, a gene-therapy extracted hemoglobin that works similarly to hemoglobin A, which is the normal adult hemoglobin. Red blood cells containing HbAT87Q have a lower risk of sickling and blocking blood flow. These modified stem cells are then transplanted into the patient. “These approvals represent an important medical advance with the use of innovative cell-based gene therapies to target potentially devastating diseases and improve public health,” writes Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research.
 Ethical considerations: Justice
Ethical considerations: Justice
The medical field has a long history of racism, and it
is important to consider that the majority of the people
affected by this disease and would be receiving CRISPR
treatment are black. Throughout the Jim Crow era in the
United States, segregation extended to healthcare
facilities, with black individuals often relegated to
substandard and underfunded hospitals and clinics. They
received inferior medical care compared to their white
counterparts, leading to stark disparities in health
outcomes. While overt forms of medical racism have
diminished over time, systemic biases and disparities
persist in healthcare systems worldwide. The use of
CRISPR in sickle cell disease treatment has the
possibility of doing immense good for a group that has
historically been neglected in the medical world.
However, if these gene therapies were to not go as
planned, this mistreated minority will be further
punished by the medical industry. Keeping these facts in
mind while considering the expansion of CRISPR SCD
treatment is crucial to the making of related major
decisions and guidelines.
Ethical considerations: Accessibility
Cost is a large topic of discussion as various sickle cell expenses accumulate to be devastatingly costly. SCD-related lifetime medical costs (blood transfusions and included inpatient care) were estimated at almost 1.7 million dollars. While the majority of this cost is covered by insurance, the disease and its implications are very time consuming. Most people have to come in to receive a blood transfusion once or even twice a month. These hospital visits are inconvenient and often get in the way of one’s day.
With that being said, one of the most critical issues about these novel CRISPR treatments is their current cost. Like most gene editing therapies, exa-cel and lovo-cell are likely to be very expensive. Estimates suggest that the price for each could be as much as $2 million per patient. Arafa Salim, who has used her own experience with sickle cell to build Tanzania’s first patient advocacy organization claimed, “A new therapy can be extremely effective, even a cure for sickle cell, but if it’s not made accessible to the average patient, it won’t be used.” Additionally, insurance companies are often motivated by a utilitarian ethic of providing the most good to the most people and could potentially deny especially expensive treatments that are deemed “experimental” and “not medically necessary,” meaning patients will have to pay out of pocket for these treatments, which is just not possible for most. As more CRISPR treatments and genetic modification are released, it is conceivable that only the wealthy will have access to this technology as far as we know. However, a one-time gene editing treatment has the potential to alleviate some of the long-term costs, including the cost of treatment, travel, and lost time or productivity for treatment. Furthermore if companies eventually get the cost of genetic therapies down and gene therapies become “standard of care”, perhaps health insurance will begin to cover CRISPR treatments. However, as of today, these therapies will not be covered by insurance for a long time as companies tend to wait as long as possible before covering a treatment, especially the more expensive ones.
Ethical considerations: Accessibility
Cost is a large topic of discussion as various sickle cell expenses accumulate to be devastatingly costly. SCD-related lifetime medical costs (blood transfusions and included inpatient care) were estimated at almost 1.7 million dollars. While the majority of this cost is covered by insurance, the disease and its implications are very time consuming. Most people have to come in to receive a blood transfusion once or even twice a month. These hospital visits are inconvenient and often get in the way of one’s day.
With that being said, one of the most critical issues about these novel CRISPR treatments is their current cost. Like most gene editing therapies, exa-cel and lovo-cell are likely to be very expensive. Estimates suggest that the price for each could be as much as $2 million per patient. Arafa Salim, who has used her own experience with sickle cell to build Tanzania’s first patient advocacy organization claimed, “A new therapy can be extremely effective, even a cure for sickle cell, but if it’s not made accessible to the average patient, it won’t be used.” Additionally, insurance companies are often motivated by a utilitarian ethic of providing the most good to the most people and could potentially deny especially expensive treatments that are deemed “experimental” and “not medically necessary,” meaning patients will have to pay out of pocket for these treatments, which is just not possible for most. As more CRISPR treatments and genetic modification are released, it is conceivable that only the wealthy will have access to this technology as far as we know. However, a one-time gene editing treatment has the potential to alleviate some of the long-term costs, including the cost of treatment, travel, and lost time or productivity for treatment. Furthermore if companies eventually get the cost of genetic therapies down and gene therapies become “standard of care”, perhaps health insurance will begin to cover CRISPR treatments. However, as of today, these therapies will not be covered by insurance for a long time as companies tend to wait as long as possible before covering a treatment, especially the more expensive ones.
"As a society, we must determine if the beneficence
outweighs the maleficence this new technology may cause.
This development uncovers an entirely new chapter of the
future of the medical field."
Conclusions and Recommendations
Given the previous discussion, the question of whether it is ethical to use CRISPR in sickle cell treatments is vastly controversial, and has seemingly unlimited arguments for and against it. As a society, we must determine if the beneficence outweighs the maleficence this new technology may cause. This development uncovers an entirely new chapter of the future of the medical field.
In order for the use of gene editing therapies to be ethical, there must be specific, strict policies from the federal government. First off, the government should prioritize equity considerations in determining access, specifically cost-wise. Individuals who cannot pay for, whose insurance does not cover these treatments, or do not live near a facility that provides CRISPR therapies should be accordingly supported. Patients must also be thoroughly informed of potential side effects that may occur from treatment. Informed consent for individuals who are not literate, do not speak the language spoken in the country they are in, and are differently abled should be adequately adapted to provide clear and fair communication of the potential risks and benefits of such treatments. And finally, there must be extensive and strict regulations to CRISPR technology in medical facilities to ensure no person or group handling these treatments goes beyond the societally decided limits. Finally, as a society we must come together and have intensive ethical discussions before further advancing CRISPR in medicine. Under these circumstances, CRISPR in medicine should and will be used to treat genetic diseases such as sickle cell disease to advance the future of humanity.
Sources:
Office of the Commissioner. “FDA Approves First Gene
Therapies to Treat Patients with Sickle Cell Disease.”
FDA, 8 Dec. 2023,
www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapies-treat-patients-sickle-cell-disease#:~:text=Casgevy%2C%20a%20cell%2Dbased%20gene.
Accessed 6 Jan. 2024.
Smith, Mike. 2023. “CRISPR.” Genome.gov. 2023. https://www.genome.gov/genetics-glossary/CRISPR.
Peebles, Angelica. 2023. “U.S. Approves First Gene-Editing Treatment, Casgevy, for Sickle Cell Disease.” CNBC. December 8, 2023. https://www.cnbc.com/2023/12/08/casgevy-first-crispr-gene-editing-treatment-approved-in-us.html.
Uddin, Fathema, et al. “CRISPR Gene Therapy: Applications, Limitations, and Implications for the Future.” Frontiers in Oncology, vol. 10, no. 1387, 7 Aug. 2020, https://doi.org/10.3389/fonc.2020.01387.
Neidler, Sarah. 2020. “Blood Transfusion - Sickle Cell Disease News.” Sickle Cell Disease News. March 5, 2020. https://sicklecellanemianews.com/blood-transfusion/#:~:text=Simple%20transfusions%20are%20typically%20given
Pagliarulo, Ned, and Shaun Lucas. “What If a CRISPR Cure Isn’t Such an Easy Choice?” BioPharma Dive, 8 Nov. 2023, www.biopharmadive.com/news/sickle-cell-crispr-gene-editing-vertex-exa-cel-barriers/698121/. Accessed 13 Jan. 2024.
Reardon, Sara. 2023. “FDA Approves First CRISPR Gene Editing Treatment for Sickle Cell Disease.” Scientific American. December 28, 2023. https://www.scientificamerican.com/article/fda-approves-first-crispr-gene-editing-treatment-for-sickle-cell-disease/#:~:text=Like%20most%20gene%20editing%20therapies.
Smith, Mike. 2023. “CRISPR.” Genome.gov. 2023. https://www.genome.gov/genetics-glossary/CRISPR.
Peebles, Angelica. 2023. “U.S. Approves First Gene-Editing Treatment, Casgevy, for Sickle Cell Disease.” CNBC. December 8, 2023. https://www.cnbc.com/2023/12/08/casgevy-first-crispr-gene-editing-treatment-approved-in-us.html.
Uddin, Fathema, et al. “CRISPR Gene Therapy: Applications, Limitations, and Implications for the Future.” Frontiers in Oncology, vol. 10, no. 1387, 7 Aug. 2020, https://doi.org/10.3389/fonc.2020.01387.
Neidler, Sarah. 2020. “Blood Transfusion - Sickle Cell Disease News.” Sickle Cell Disease News. March 5, 2020. https://sicklecellanemianews.com/blood-transfusion/#:~:text=Simple%20transfusions%20are%20typically%20given
Pagliarulo, Ned, and Shaun Lucas. “What If a CRISPR Cure Isn’t Such an Easy Choice?” BioPharma Dive, 8 Nov. 2023, www.biopharmadive.com/news/sickle-cell-crispr-gene-editing-vertex-exa-cel-barriers/698121/. Accessed 13 Jan. 2024.
Reardon, Sara. 2023. “FDA Approves First CRISPR Gene Editing Treatment for Sickle Cell Disease.” Scientific American. December 28, 2023. https://www.scientificamerican.com/article/fda-approves-first-crispr-gene-editing-treatment-for-sickle-cell-disease/#:~:text=Like%20most%20gene%20editing%20therapies.


